 | |
| Names | |
|---|---|
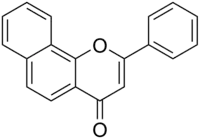
| IUPAC name
Benzo[7,8]flavone | |
| Systematic IUPAC name
2-Phenyl-4H-naphtho[1,2-b]pyran-4-one | |
| Other names
7,8-Benzoflavone ANF 2-Phenyl-benzo[h]chromen-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.009.156 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H12O2 | |
| Molar mass | 272.303 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
α-Naphthoflavone, also known as 7,8-benzoflavone and 2-phenyl-benzo[h]chromen-4-one, is a synthetic[1][2] flavone derivative. It can be prepared from 2-naphthol and cinnamaldehyde.[3]
α-Naphthoflavone is a potent inhibitor of the enzyme aromatase, the enzyme that converts testosterone to estrogen.[1][2] α-Naphthoflavone has been shown to cause abnormal testicular development in young chickens.[4]
See also
References
- 1 2 Campbell, Deborah R.; Kurzer, Mindy S. (1993). "Flavonoid inhibition of aromatase enzyme activity in human preadipocytes". Journal of Steroid Biochemistry and Molecular Biology. 46 (3): 381–388. doi:10.1016/0960-0760(93)90228-O. PMID 9831487. S2CID 25861427.
- 1 2 Kellis JT Jr; Vickery LE (1984). "Inhibition of human estrogen synthetase (aromatase) by flavones". Science. 225 (4666): 1032–1034. Bibcode:1984Sci...225.1032K. doi:10.1126/science.6474163. PMID 6474163.
- ↑ Harvey, Ronald G.; Hahn, Jung Tai; Bukowska, Maria; Jackson, Henry (1990). "A new chromone and flavone synthesis and its utilization for the synthesis of potentially antitumorigenic polycyclic chromones and flavones". The Journal of Organic Chemistry. 55 (25): 6161. doi:10.1021/jo00312a023.
- ↑ Trefil, P.; Micakova, A.; Stiborova, M.; Poplstein, M.; Brillard, J.P.; Hodek, P. (2004). "Effects of alpha-naphthoflavone on body growth and gonad development in chickens (Gallus domesticus)". Czech Journal of Animal Science. 49 (6): 231–238. doi:10.17221/4305-CJAS.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.