 | |
 | |
 | |
| Names | |
|---|---|
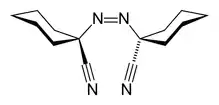
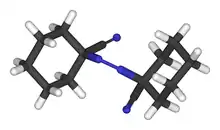
| Systematic IUPAC name
1,1′-Diazene-1,2-diyldicyclohexanecarbonitrile | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | ACHN |
| 960744 | |
| ChemSpider | |
| ECHA InfoCard | 100.016.595 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 3226 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H20N4 | |
| Molar mass | 244.342 g·mol−1 |
| Melting point | 114 to 118[1] °C (237 to 244 °F; 387 to 391 K) decomposes near 80 °C |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H242, H315, H319, H335 | |
| P261, P305+P351+P338 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,1′-Azobis(cyclohexanecarbonitrile) or ACHN is a radical initiator.[1] The molecular formula is NCC6H10N=NC6H10CN. It is a white solid that is soluble in aromatic solvents.[2]
See also
- Azobisisobutylonitrile (AIBN) is another commonly used free radical initiator
References
- 1 2 3 1,1′-Azobis(cyclohexanecarbonitrile) at Sigma-Aldrich
- ↑ Steven A. Kates, Fernando Albericio (2001). "1,1'-Azobis-1-cyclohexanenitrile". E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ra120.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.