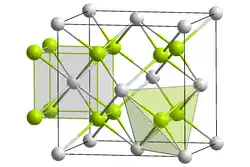
 Crystal form of californium oxyfluoride __ Cf3+ __ O2− or F− | |
| Names | |
|---|---|
| IUPAC name
Californium(III) oxyfluoride | |
| Identifiers | |
3D model (JSmol) |
|
| |
| |
| Properties | |
| CfFO | |
| Molar mass | 286 g·mol−1 |
| Structure | |
| cubic | |
a = 556.1 ± 0.4 pm | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Californium(III) oxyfluoride is a radioactive inorganic compound with a chemical formula CfOF, synthesized in the 1960s. This salt crystallizes with the cubic fluorite structure, with the oxide and fluoride anions randomly distributed in anion sites.[1]
Synthesis
Californium(III) oxyfluoride is an oxyfluoride and a mixed anion compound. It can be prepared by the hydrolysis of CfF3 at high temperature.[1]
References
- 1 2 Peterson, J.R.; Burns, John H. (November 1968). "Preparation and crystal structure of californium oxyfluoride, CfOF". Journal of Inorganic and Nuclear Chemistry. 30 (11): 2955–2958. doi:10.1016/0022-1902(68)80155-1.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.