 | |
| Names | |
|---|---|
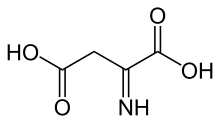
| IUPAC name
2-Iminobutanedioic acid | |
| Other names
Iminoaspartic acid; 2-iminobutanedioic acid, iminosuccinate, alpha-iminosuccinate, iminosuccinic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C4H5NO4 | |
| Molar mass | 131.087 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Iminoaspartic acid (also known as iminosuccinate or iminoaspartate) is a dicarboxylic acid in the biosynthesis of nicotinic acid. It is synthesised by the oxidation of aspartate and is condensed by quinolinate synthase with glycerone phosphate to form quinolinic acid.[1]
References
- ↑ Ollagnier-de Choudens, S., Loiseau, L., Sanakis, Y., Barras, F. and Fontecave, M. (2005). "Quinolinate synthetase, an iron-sulfur enzyme in NAD biosynthesis" (PDF). FEBS Lett. 579 (17): 3737–3743. doi:10.1016/j.febslet.2005.05.065. PMID 15967443.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.