 | |
| Names | |
|---|---|
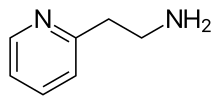
| Preferred IUPAC name
2-(Pyridin-2-yl)ethan-1-amine | |
| Identifiers | |
3D model (JSmol) |
|
| 111208 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.450 |
| EC Number |
|
| MeSH | 2-(2-Aminoethyl)pyridine |
PubChem CID |
|
| UNII | |
| UN number | 2735 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H10N2 | |
| Molar mass | 122.171 g·mol−1 |
| Density | 1.021 g cm−3 |
| Boiling point | 93 °C; 199 °F; 366 K at 1.6 kPa |
| log P | -0.11 |
Refractive index (nD) |
1.536 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P305+P351+P338 | |
| NFPA 704 (fire diamond) | |
| Flash point | 100 °C (212 °F; 373 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Pyridylethylamine is a histamine agonist which is selective for the H1 subtype.[1]
References
- ↑ Flynn SB, Gristwood RW, Owen DA (January 1979). "Differentiation of the roles of histamine H1- and H2-receptors in the mediation of the effects of histamine in the isolated working heart of the guinea-pig". Br. J. Pharmacol. 65 (1): 127–37. doi:10.1111/j.1476-5381.1979.tb17341.x. PMC 1668480. PMID 32943.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
