 | |
| Names | |
|---|---|
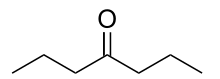
| Preferred IUPAC name
Heptan-4-one | |
| Other names
Dipropyl ketone, Butyrone, DPK, Propyl ketone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.191 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2710 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H14O | |
| Molar mass | 114.188 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.82 g/mL |
| Melting point | −32.8 °C (−27.0 °F; 240.3 K) |
| Boiling point | 143.9 °C (291.0 °F; 417.0 K) |
| Vapor pressure | 5 mmHg (20°C) |
| -80.45·10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Combustible[1] |
| GHS labelling: | |
  | |
| Warning | |
| H226, H332 | |
| P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P304+P312, P304+P340, P312, P370+P378, P403+P235, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 48.9 °C (120.0 °F; 322.0 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
TWA 50 ppm (235 mg/m3)[1] |
IDLH (Immediate danger) |
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
4-Heptanone or heptan-4-one is an organic compound with the formula (CH3CH2CH2)2CO. It is a colorless liquid.
Synthesis
The compound is synthesized by ketonization, involving the pyrolysis of iron(II) butyrate. [2]
Butyrone is used in the synthesis of 3-propylthio-4-heptanol [1838-73-9],[3] which has found use as a flavor augmenting or enhancing composition in foodstuffs.
References
- 1 2 3 4 NIOSH Pocket Guide to Chemical Hazards. "#0242". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Davis, Robert; Granito, Charles; Schultz, Harry P. (1967). "4-Heptanone". Organic Syntheses. 47: 75. doi:10.15227/orgsyn.047.0075.
- ↑ William J. Evers, et al. U.S. Patent 4,097,615 (1978 to International Flavors and Fragrances Inc).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
