Bromochlorobenzene is any of three different positional isomers consisting of a bromine atom and a chlorine atom as substituents on a benzene ring.
| Isomers of Bromochlorobenzene | |||
|---|---|---|---|
| Skeletal formula | 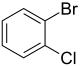 |
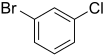 |
|
| General | |||
| Common names | o-bromochlorobenzene ortho-bromochlorobenzene |
m-bromochlorobenzene meta-bromochlorobenzene |
p-bromochlorobenzene para-bromochlorobenzene |
| Systematic name | 1-bromo-2-chlorobenzene | 1-bromo-3-chlorobenzene | 1-bromo-4-chlorobenzene |
| Molecular formula | BrC6H4Cl | ||
| Molar mass | 191.45 g/mol | ||
| CAS number | 694-80-4 | 108-37-2 | 106-39-8 |
| ChemSpider | 12230 | 13875377 | 7518 |
| PubChem CID | 12754 | 7928 | 7806 |
| Properties | |||
| Melting point | −13 °C (260 K) | −22 °C (251 K) | 63–67 °C (336–340 K) |
| Boiling point | 203–205 °C (476–478 K) | 195–196 °C (468–469 K) | 196 °C (469 K) |
All three have been synthesized by various routes:
- 1-Bromo-2-chlorobenzene: from 2-chloroaniline, via diazotization followed by a Sandmeyer reaction[1]
- 1-Bromo-3-chlorobenzene: by (3-chlorophenyl)trimethylgermanium by electrophilic substitution[2]
- 1-Bromo-4-chlorobenzene:
- From a derivative of (4-bromophenyl)silane using N-bromosuccinimide[3]
- From 4-chlorophenol using triphenylphosphine dibromide[4] or phenylphosphorus tetrachloride[5]
References
- ↑ Hartwell, Jonathan L. (1944). "o-Chlorobromobenzene". Organic Syntheses. 24: 22.
- ↑ Moerlein, S. M. (1987). "Use of aryltrimethylgermanium substrates for facile aromatic chlorination, bromination, and iodination". The Journal of Organic Chemistry. 52 (4): 664–667. doi:10.1021/jo00380a031.
- ↑ Hosomi, Akira; Iijima, Susumu; Sakurai, Hideki (1981). "Carbon–silicon bond cleavage of organotrialkoxysilanes and organosilatranes with m-chloroperbenzoic acid and N-bromosuccinimide. New route to phenols, primary alcohols and bromides". Chemistry Letters. 10 (2): 243–246. doi:10.1246/cl.1981.243.
- ↑ Wiley, G. A.; Hershkowitz, R. L.; Rein, B. M.; Chung, B. C. (1964). "Studies in Organophosphorus Chemistry. I. Conversion of Alcohols and Phenols to Halides by Tertiary Phosphine Dihalides". Journal of the American Chemical Society. 86 (5): 964–965. doi:10.1021/ja01059a073.
- ↑ Bay, Elliott; Bak, David A.; Timony, Peter E.; Leone-Bay, Andrea (1990). "Preparation of aryl chlorides from phenols". The Journal of Organic Chemistry. 55 (10): 3415–3417. doi:10.1021/jo00297a087.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.