 | |
| Names | |
|---|---|
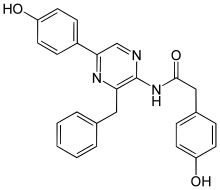
| Preferred IUPAC name
N-[3-Benzyl-5-(4-hydroxyphenyl)pyrazin-2-yl]-2-(4-hydroxyphenyl)acetamide | |
| Other names
Coelenteramide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C25H21N3O3 | |
| Molar mass | 411.461 g·mol−1 |
| Density | 1.26 g/cm3 |
| Absorbance | ε332.5 = 15000 M−1 cm−1 (methanol)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Coelenteramide is the oxidized product, or oxyluciferin, of the bioluminescent reactions in many marine organisms that use coelenterazine. It was first isolated as a blue fluorescent protein from Aequorea victoria after the animals were stimulated to emit light.[2] Under basic conditions, the compound will break down further into coelenteramine and 4-hydroxyphenylacetic acid.
It is an aminopyrazine.[3]
References
- ↑ Shimomura, Osamu (2012). Bioluminescence : chemical principles and methods. Singapore Hackensack, NJ: World Scientific Publishing Co. Pte. Ltd. ISBN 978-981-4366-08-3. OCLC 794263013.
- ↑ Shimomura O, Johnson FH (1975). "Chemical Nature of Bioluminescence Systems in Coelenterates". PNAS USA. 72 (4): 1546–1549. doi:10.1073/pnas.72.4.1546. PMC 432574. PMID 236561.
- ↑ Discovery and Validation of a New Family of Antioxidants: The Aminopyrazine Derivatives. M. L. N. Dubuisson, J.-F. Rees and J. Marchand-Brynaert, Mini-Reviews in Medicinal Chemistry, 2004, 4, 159-165, doi:10.2174/1389557043403927
External links
 Media related to Coelenteramide at Wikimedia Commons
Media related to Coelenteramide at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.