 | |
| Names | |
|---|---|
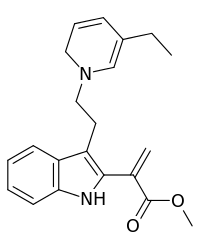
| Preferred IUPAC name
Methyl 2-{3-[2-(5-ethylpyridin-1(2H)-yl)ethyl]-1H-indol-2-yl}prop-2-enoate | |
| Other names
Methyl 2-{3-[2-(5-ethyl-1(2H)-pyridinyl)ethyl]-1H-indol-2-yl}acrylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C21H24N2O2 | |
| Molar mass | 336.435 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dehydrosecodine is a terpene indole alkaloid. The compound is believed to be an unstable O-acetylated secodine type intermediate in the formation of catharanthine and tabersonine from stemmadenine.[1] The enzymes involved forming dehydrosecodine or utilizing it as a substrate for further chemical reactions are currently unknown.
References
- ↑ Qu, Y., Easson, M. E., Simionescu, R., Hajicek, J., Thamm, A. M., Salim, V., & De Luca, V. (2018). Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine. Proceedings of the National Academy of Sciences, 115(12), 3180-3185.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.