 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
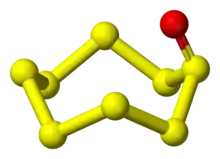
| S8O | |
| Molar mass | 272.48 g·mol−1 |
| Appearance | Yellowish sharp crystal[1] |
| Density | 2.13 g·cm−3 |
| Solubility | 0.8 g(carbon disulfide)[1] |
| Structure[2] | |
| Orthorhombic | |
| Pca21 | |
a = 13.197, b = 7.973, c = 8.096 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Octasulfur monoxide is an inorganic compound with a chemical formula S8O, discovered in 1972. It is a type of sulfur oxide.[1]
A crystalline compound composed of cyclooctasulfur monoxide and antimony pentachloride in equimolar quantities can be made (S8O·SbCl5).[3]
References
- 1 2 3 Ralf Steudel, Michael Rebsch (April 1972). "Preparation of cyclo-Octasulfur Oxide, S8O". Angewandte Chemie International Edition in English. 11 (4): 302–303. doi:10.1002/anie.197203021. ISSN 0570-0833.
- ↑ Steudel, Ralf; Luger, Peter; Bradaczek, Hans; Rebsch, Michael (May 1973). "Crystal and Molecular Structure of Cyclooctasulfur Oxide, S8O". Angewandte Chemie International Edition in English. 12 (5): 423–424. doi:10.1002/anie.197304231.
- ↑ Steudel, Ralf; Sandow, Torsten; Steidel, Jürgen (1980). "Reversible isomerization of cyclo-octasulphur monoxide; preparation and X-ray crystal structure of S8O·SbCl5". J. Chem. Soc., Chem. Commun. (4): 180–181. doi:10.1039/C39800000180.
External links
 Media related to Octasulfur monoxide at Wikimedia Commons
Media related to Octasulfur monoxide at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.