 | |
| Clinical data | |
|---|---|
| Other names | Probarbital, Ipral, Vasalgin, 5-Isopropyl-5-ethylbarbituric acid |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
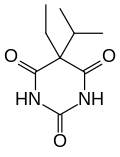
| Formula | C9H14N2O3 |
| Molar mass | 198.222 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Probarbital (trade names Ipral, Vasalgin) is a barbiturate derivative invented in the 1920s. It has sedative, hypnotic and anticonvulsant properties.
References
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.