 | |
| Names | |
|---|---|
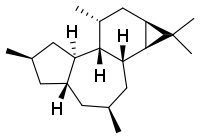
| Preferred IUPAC name
(1aS,1bR,3S,4aS,6R,7aR,7bR,8R,9aR)-1,1,3,6,8-pentamethyltetradecahydro-1H-cyclopropa[3,4]benzo[1,2-e]azulene | |
| Other names
Tiglian | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H34 | |
| Molar mass | 274.492 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tigliane is a diterpene that forms the structural basis for some natural chemical compounds such as phorbol.[1][2]
See also
- Abietane
- Labdane
- Ingenane
- Phorbol esters
- Tigilanol tiglate
References
- ↑ "Tigliane", Pubchem, retrieved 2022-08-08
- ↑ Qi-Run Li, Yung-Yi Cheng, Lei Zhao, Xiao-Lei Huang, Xiao-Gang Jiang, Ya-Dong Cui, Susan L. Morris-Natschke, Masuo Goto, Chin-Ho Chen, Kuo-Hsiung Lee, Dao-Feng Chen, Jian Zhang, New phorbol ester derivatives as potent anti-HIV agents, Bioorganic & Medicinal Chemistry Letters, 50, 2021, doi:10.1016/j.bmcl.2021.128319; ISSN 0960-894X.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.